The main purpose of this ongoing blog will be to track planetary extreme, or record temperatures related to climate change. Any reports I see of ETs will be listed below the main topic of the day. I’ll refer to extreme or record temperatures as ETs (not extraterrestrials).😉
Main Topic: Turning Plastic Waste into a Carbon Sync
Dear Diary. Today’s main topic seems like it is too good to be true. What if some plastic wastes could actually be treated such that they soak up carbon going into the atmosphere? Indeed, that is what researchers at Rice University have discovered. While not solving the climate crisis, if plastics were manufactured to soak up carbon, this would go a long way towards that goal.
Here are more details on these groundbreaking findings:
Turning Plastic Waste Into Carbon-Capture Master That Can Soak Up Excess Carbon Dioxide
TOPICS: Carbon Capture Carbon Dioxide Nanotechnology Plastic Popular Rice University
By RICE UNIVERSITY APRIL 6, 2022
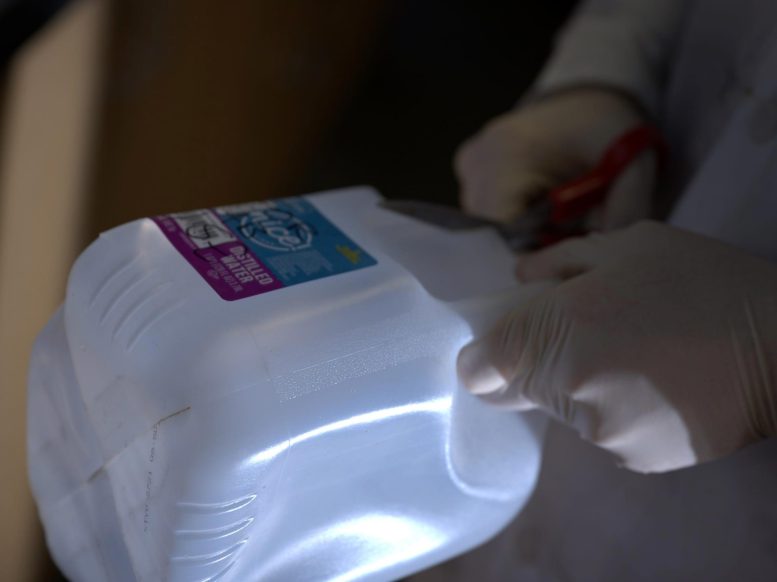
A plastic jug is fodder for a material developed at Rice University that turns waste plastic into a material that absorbs carbon dioxide. The lab is targeting flue gases that now require a far more complex process to sequester carbon dioxide. Credit: Jeff Fitlow/Rice University
Rice University lab turns hard-to-process trash into carbon-capture master.
Here’s another thing to do with that mountain of used plastic: make it soak up excess carbon dioxide.
What seems like a win-win for a pair of pressing environmental problems describes a Rice University lab’s newly discovered chemical technique to turn waste plastic into an effective carbon dioxide (CO2) sorbent for industry.
Rice chemist James Tour and co-lead authors Rice alumnus Wala Algozeeb, graduate student Paul Savas and postdoctoral researcher Zhe Yuan reported in the American Chemical Society journal ACS Nano that heating plastic waste in the presence of potassium acetate produced particles with nanometer-scale pores that trap carbon dioxide molecules.
These particles can be used to remove CO2 from flue gas streams, they reported.

Rice University graduate student Paul Savas feeds raw plastic into a crusher to prepare it for pyrolysis, or heating in an inert atmosphere. Pyrolyzing the material in the presence of potassium salts turns it into a material that sequesters carbon dioxide from flue gas. Credit: Jeff Fitlow/Rice University
“Point sources of CO2 emissions like power plant exhaust stacks can be fitted with this waste-plastic-derived material to remove enormous amounts of CO2 that would normally fill the atmosphere,” Tour said. “It is a great way to have one problem, plastic waste, address another problem, CO2 emissions.”
A current process to pyrolyze plastic known as chemical recycling produces oils, gases and waxes, but the carbon byproduct is nearly useless, he said. However, pyrolyzing plastic in the presence of potassium acetate produces porous particles able to hold up to 18% of their own weight in CO2 at room temperature.

Pores in this micron-scale particle, the result of pyrolyzing in the presence of potassium acetate, are able to sequester carbon dioxide from streams of flue gas. Rice University scientists say the process could be a win-win for a pair of pressing environmental problems. Credit: Tour Group/Rice University
In addition, while typical chemical recycling doesn’t work for polymer wastes with low fixed carbon content in order to generate CO2 sorbent, including polypropylene and high- and low-density polyethylene, the main constituents in municipal waste, those plastics work especially well for capturing CO2 when treated with potassium acetate.
The lab estimates the cost of carbon dioxide capture from a point source like post-combustion flue gas would be $21 a ton, far less expensive than the energy-intensive, amine-based process in common use to pull carbon dioxide from natural gas feeds, which costs $80-$160 a ton.

A Rice University chemist prepares to heat plastic powder combined with potassium acetate to turn it into porous particles that absorb carbon dioxide. Credit: Jeff Fitlow/Rice University
Like amine-based materials, the sorbent can be reused. Heating it to about 75 degrees Celsius (167 degrees Fahrenheit) releases trapped carbon dioxide from the pores, regenerating about 90% of the material’s binding sites.
Because it cycles at 75 degrees Celsius, polyvinyl chloride vessels are sufficient to replace the expensive metal vessels that are normally required. The researchers noted the sorbent is expected to have a longer lifetime than liquid amines, cutting downtime due to corrosion and sludge formation.

Rice University chemists modify waste plastic to absorb carbon dioxide from flue gas streams more efficiently than current processes. From left: Paul Savas, James Tour and Zhe Yuan. Credit: Jeff Fitlow/Rice University
To make the material, waste plastic is turned into powder, mixed with potassium acetate and heated at 600 C (1,112 F) for 45 minutes to optimize the pores, most of which are about 0.7 nanometers wide. Higher temperatures led to wider pores. The process also produces a wax byproduct that can be recycled into detergents or lubricants, the researchers said.
Reference: “Plastic Waste Product Captures Carbon Dioxide in Nanometer Pores” by Wala A. Algozeeb, Paul E. Savas, Zhe Yuan, Zhe Wang, Carter Kittrell, Jacklyn N. Hall, Weiyin Chen, Praveen Bollini and James M. Tour, 5 April 2022, ACS Nano.
DOI: 10.1021/acsnano.2c00955
Co-authors of the paper are Rice alumnus Zhe Wang and research scientist Carter Kittrell, and graduate student Jacklyn Hall and Praveen Bollini, an assistant professor of chemical and biomolecular engineering, both of the University of Houston. Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of materials science and nanoengineering.
The Department of Energy (DE-F0031794) and Saudi Aramco supported the research.
We recommend:
- Cheaper Carbon Capture Is on the Way – Marathon Research Effort Drives Down Cost Mike ONeill, SciTechDaily, 2021
- Hexagonal Boron Nitride’s Incredible Toughness Unmasked – “What We Observed … Is Remarkable! “Mike ONeill, SciTechDaily, 2021
- 3D-Printed Device Enhances Capture of Carbon Dioxide Emissions Mike ONeill, SciTechDaily, 2020
- Carbon Capture Gets Cheaper: Making Methane From CO2Mike ONeill, SciTechDaily, 2021
- Solar Energy Breakthrough: Ultrathin Solar Cells Using 2D Perovskites Get a Boost Mike ONeill, SciTechDaily, 2021
- New metal organic framework can produce valuable chemicals out of factory smoke Alexandru Micu, ZME Science, 2022
- GEOLOGIC SEQUESTRATION OF CO2 AND FLUE GASES INTO DEEP AUSTRALIAN COAL SEAMS WITH ENHANCED COALBED METHANE RECOVERY World Scientific Book
- Electricity turns garbage into high-quality graphene Robert F. Service, Science, 2020
- Flue Gas Desulfurization by Mechanically and Thermally Activated Sodium Bicarbonate Barbara Walawska et al., Polish Journal of Chemical Technology, 2014
- Submit Today! Special Issue: “Advances in Proximal Sensing and Image Analysis for Time-Series Phenotyping” Plant Phenomics, 2021
Related:
Here are some “ET’s” recorded over the last couple of days:
Here is some more March 2022 climatology:
Here is more climate and weather news from Tuesday:
(As usual, this will be a fluid post in which more information gets added during the day as it crosses my radar, crediting all who have put it on-line. Items will be archived on this site for posterity. In most instances click on the pictures of each tweet to see each article. The most noteworthy items will be listed first.)
Now here are some of today’s articles and notes on the horrid war on Ukraine:
(If you like these posts and my work please contribute via the PayPal widget, which has recently been added to this site. Thanks in advance for any support.)
Guy Walton “The Climate Guy”